Abstract
Background:Reduced-intensity conditioning (RIC) regimens such as Flu/Bu and Flu/Mel are commonly used for patients who are not medically fit for myeloablative conditioning (Shimoni Leukemia 2006). Recent studies have shown lower incidences of non-relapse mortality (NRM), acute graft-versus-host disease (aGVHD), and toxicity with Flu/Bu using 2 days of busulfan, whereas Flu/Mel has been associated with longer overall (OS) and relapse-free survival (RFS) (Shimoni Leukemia 2007, Baron Cancer 2015, Damlaj BBMT 2016). It is unknown whether use of more intense Flu/Bu regimens result in similar outcomes and toxicity profiles when compared to Flu/Mel. We sought to compare the toxicities and outcomes between patients receiving Flu/Bu using either three or four days of busulfan (Flu/Bu3 and Flu/Bu4, respectively) and Flu/Mel.
Methods:We retrospectively evaluated patients with hematologic malignancies who underwent allo-HCT between 2010 and 2015 with Flu/Mel or Flu/Bu as their conditioning regimen. Busulfan was pharmacokinetically (PK) dosed, with a target area under the curve of 4,700 (range 4,100 - 5,200) μmol*min/L. We collected all grade ≥ 3 non-hematologic toxicities from the start of the conditioning regimen up to day +365 or relapse/death, and organized them into organ-based categories. OS and RFS were estimated using the Kaplan-Meier method with differences assessed with a log-rank test. The cumulative incidence method for competing risks was used in estimating non-relapse mortality, GVHD, as well as the incidence of individual toxicities, with differences assessed with Gray's test.
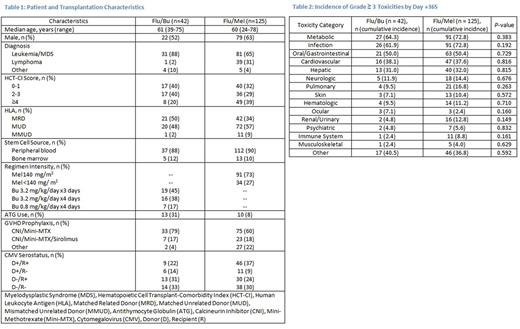
Results: We included 167 patients in this toxicity analysis: 42 patients received Flu/Bu and 125 patients received Flu/Mel. Baseline patient and HCT characteristics are shown in Table 1. The most common GVHD prophylaxis in both arms was tacrolimus and mini-methotrexate. Cytomegalovirus (CMV) serostatus was similar in both arms. More patients receiving Flu/Bu had acute leukemia/myelodysplastic syndrome, matched related donors, and lower hematopoietic cell transplant-comorbidity index scores. The median time to neutrophil (12 days vs 13 days) and platelet engraftment (16 days vs 20 days) was similar between Flu/Bu and Flu/Mel, respectively. Though there were no statistically significant differences in the incidence of toxicities by category between Flu/Bu and Flu/Mel (Table 2), certain toxicities varied. Despite a higher proportion of patients in the Flu/Bu arm receiving antithymocyte globulin, patients receiving Flu/Mel had a higher incidence of infections such as bacteremia (20% vs 5%), CMV reactivation (16% vs 7%), and sepsis (16% vs 0%). Patients receiving Flu/Mel also had more metabolic derangements, specifically hypophosphatemia (51% vs 36%), hypocalcemia (40% vs 26%), hypokalemia (30% vs 17%), hyponatremia (28% vs 14%), and anorexia requiring total parenteral nutrition (17% vs 5%), compared to those receiving Flu/Bu. Rates of grade ≥ 3 mucositis (31% vs 31%), nausea/vomiting (21% vs 17%), and diarrhea (19% vs 21%) were similar for Flu/Bu and Flu/Mel, respectively. Patients receiving Flu/Bu were more likely to experience grade ≥ 3 AST (40% vs 26%) and ALT (29% vs 21%) elevations. However, veno-occlusive disease was rare, occurring in 2 patients who received Flu/Bu and 1 patient receiving Flu/Mel. There was a trend toward a lower incidence of aGVHD in patients receiving Flu/Bu compared to Flu/Mel (31% vs 48% at day 100, respectively, p=0.055). There was no significant difference in NRM (4.8% vs 14.4% at 1 year, p=0.234), OS (78.6% vs 66.9% at 1 year, p=0.15) or RFS (59.5% vs 48.8% at 1 year, p=0.15) between Flu/Bu and Flu/Mel, respectively.
Conclusions: Our results showed similar survival, incidence of aGVHD, and specific organ toxicities between more intense Flu/Bu regimens and Flu/Mel. Our analysis suggests that utilizing PK-dosed busulfan to target more intense Flu/Bu regimens can maximize disease control without increasing serious toxicities. Moreover, extending a PK-dosing strategy to melphalan may similarly achieve favorable outcomes while minimizing toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal